Several nanoparticle-based technologies have been successfully used for in vivo gene delivery, including chemically synthesized nanoparticles, inorganic nanoparticles (gold, silica, calcium phosphates) and quantum dots consisting of nanotubes or nanorods. Nanoparticles bring forth their distinctive characteristics as a means to improve stability, biodistribution, high surface to volume ratio, solubility, half-life and tissue specificity of liposomes.
NANOPARTICLE TRANSFECTION COMPLEXES
Nanoparticle transfection commonly uses small metal particles such as gold (Au) and metal functionalized particles to aid in the process as a vehicle for delivering active pharmaceutical ingredients (API) to the cell or tissue. Nanoparticles can be utilized for transfection of cancer cell lines, primary cultures, as well as tissue targeting and in vivo delivery. Typically, a nanoparticle consists of two parts:
- Inorganic core: gold metallic core; tunable monodispersed nanoparticles, core materials are nontoxic and inert, gold particle enables efficient conjugation of the API and targeting ligand
- Organic monolayer: targeting ligands, API, liposomes
Current limits of treating genetic disorders is delivering a gene therapy into a living cell. Nanoparticles provide the necessary framework and stability to be an efficient gene delivery system. These requirements include altering the metallic core size and shape. The functionality of the gold particle surface enables formulation changes of active groups, which results in alternate interactions with the payload. Altering the overall monolayer charge and length, along with payload to particle ratios govern delivery success.
Commercially available nanoparticle reagents and kits are available – In Vivo Nanoparticle Kit
NANOPARTICLE IN VIVO TRANSFECTION PROTOCOL
NANOPARTICLE LIPOSOME DESCRIPTION
Nanoparticle liposome-based In Vivo Transfection Reagent is an animal-origin-free, proprietary formulation containing chemically engineered nanoparticles optimized for in vivo delivery of siRNA, miRNA, shRNA and plasmid DNA. Nanoparticle complexes are stable for at least 16 hours in serum and have minimal toxicity. Efficient delivery has been exhibited for siRNA and plasmid DNA to these tissues: pancreas, kidney, liver, lung and tumors via systemic tail vein administration.
NANOPARTICLE LIPOSOME SYSTEMIC ADMINISTRATION (I.V. INJECTION)
Below is a protocol for the i.v. injection of nanoparticles for in vivo experiments.
- Dilute 100 µg of plasmid DNA or 60 µg of siRNA in 100 µL nuclease-free water and vortex gently.
- Add 100 µL of the diluted to a sterile tube containing 50 µL Transfection Reagent.
- Incubate for 15-20 min at room temperature.
- Add 10 µL of Transfection Enhancer Reagent and vortex gently to mix.
- Incubate for 5 min at room temperature.
- Add required amount of sterile solution of 5% glucose (w/v):
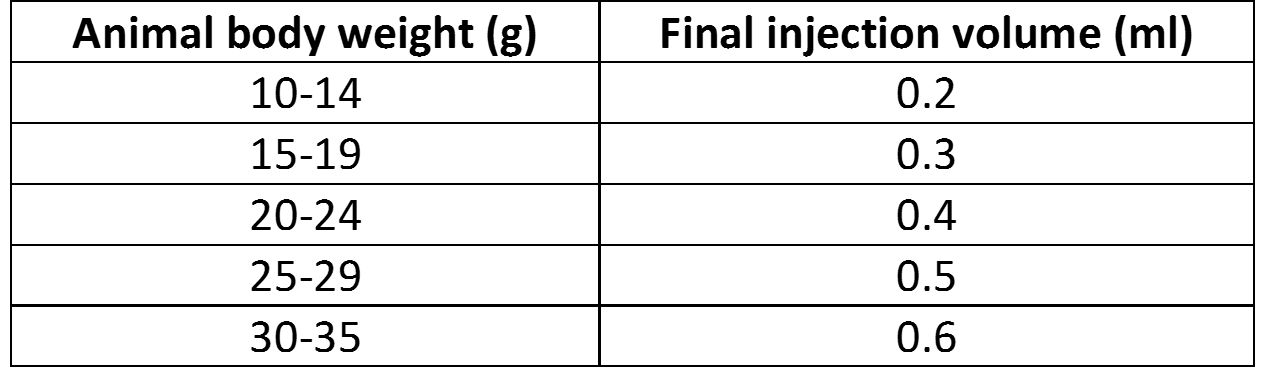
- Inject animals: Delivery efficiency can be increased with a secondary injection.
- Efficacy: Maximum mRNA target effect is observed 12-36 hours after injection, with maximum effect on protein level achieved 24-48 hours post-injection.
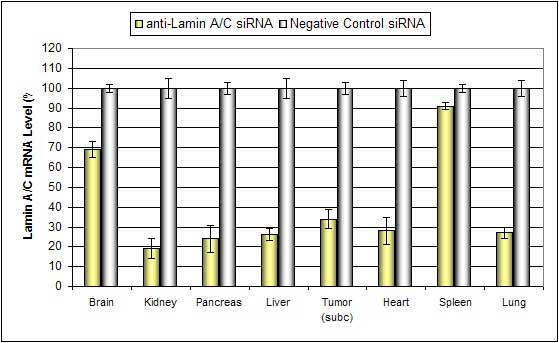
Altogen Biosystems manufactures nanoparticle-, liposome-, and polymer-based in vivo products and services, as well as tissue-targeted in vivo transfection reagents and kits:
- Liver-targeted in vivo transfection kit
- Kidney-targeted in vivo transfection kit
- Pancreas-targeted in vivo transfection kit
IN VIVO TRANSFECTION | PEG LIPOSOME TRANSFECTION | NANOPARTICLE TRANSFECTION | LIPID-BASED TRANSFECTION | POLYMER-BASED TRANSFECTION | USES OF DNA TRANSFECTION