POLYMER TRANSFECTION

Transfection of cell lines can be performed using polymers that are able to form a complex with cargo molecules (i.e. RNAi or small protein) and form aggregates with surface elements on the target cell membrane. This reaction results in cargo intracellular delivery occurs in aqueous solution that allows polymer entry.
Transfection (or transduction) is a process of introducing foreign molecules into a living cell to modify its function or cure certain disease. In biology research applications transfection method often utilize polymers for transfection of eukaryotic cells (animal, plant, and fungus cells) with plasmid DNA (encoding gene of interest) or siRNA (targeting mRNA of gene of interest).
POLYMER-BASED COMPLEXES
Polymer-based transfection works similar to lipid-based transfection. Polymers are usually an artificially created structure of chemical synthesis. While polymer-based transfection approach can be useful for transfection on certain types of cells, it is also known to be resistant to other forms.
Synthetic polymers offer the advantage of exhibiting extremely low immune response and ease of tuning their chemical and physical properties. The polymer complex compacts its cargo (DNA, miRNA, siRNA) into viral-sized particles that are able to navigate through the circulatory system and associate with the targeted tissue. While in circulation, the polymer-based transfection complex provides degradation protection to the payload and inhibits biological interactions that could result in immune responses. However, the mode in which polymer-based transfection reagents deliver their cargo to cells is unknown.
As with any transfection reagent, optimization and particle formation should be optimized. There are many areas of consideration, such as:
- Concentration of the payload
- Ionic strength of the complex
- Net charge (positive to negative) of the polymer and cargo
- Complex formation process; such as thermodynamic vs kinetic vs mechanical
- Polymer branching; branched vs linear polymer
- Polycation; polyornithine, histones, polylysine, polyphosphoramidates, polyethylenimine
- Payload modifications
In vivo polymer transfection reagents are commercially available from manufacturer (Altogen Biosystems).
POLYMER-BASED IN VIVO TRANSFECTION PROTOCOL
POLYMER-BASED LIPOSOME DESCRIPTION
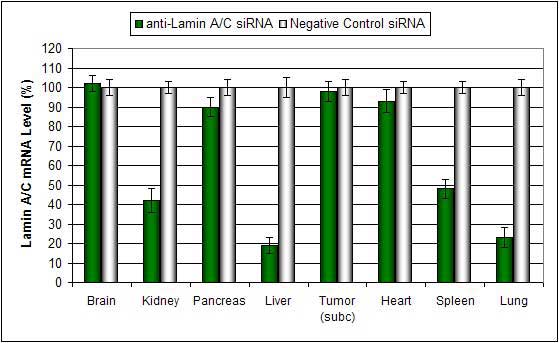
Polymer-based transfection reagent is a biodegradable polymer-based formulation optimized for in vivo delivery of siRNA, microRNA, shRNA and plasmid DNA. Polymer conjugated complexes can be stable in serum for at least 16 hours. Efficient delivery to the lung, liver, kidney and certain tumor types via systemic administration with minimal toxicity. Efficient siRNA and plasmid DNA delivery has been demonstrated via direct subcutaneous tumor injection.
Modes of Administration:
- Tail vein systemic intravenous (i.v.) injection
- Direct intratumoral (i.t.) injection.
POLYMER-BASED SYSTEMIC ADMINISTRATION
Below is a protocol for the intravenous injection of polymer-based complexes for in vivo experiments.
- Dilute 100 µg of siRNA/miRNA or 60 µg of plasmid DNA in 100 µL of nuclease-free water and vortex gently.
- Add 100 µL of the diluted nucleic acid to a sterile tube containing 50 µL Transfection Reagent.
- Allow the complexes to incubate for 15-20 min at room temperature.
- Add 10 µL of the Transfection Enhancer Reagent to the tube and vortex gently to mix.
- Incubate for 5 min at room temperature.
- Add required amount of sterile solution of 5% glucose (w/v):
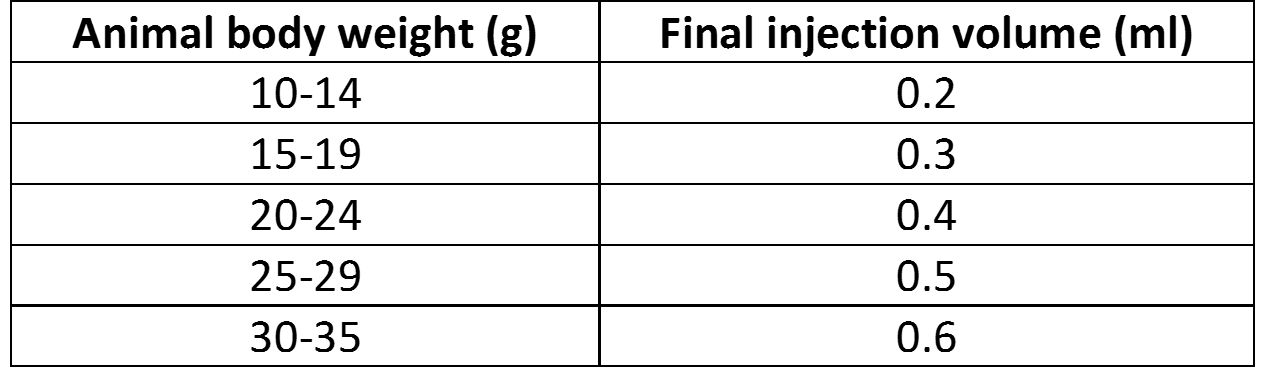
- Inject animals; optional: delivery efficiency can be increased with a secondary injection.
- Efficacy: Maximum mRNA target effect is observed 12-36 hours after injection, with maximum effect on protein level achieved 24-48 hours post-injection.
IN VIVO TRANSFECTION | PEG LIPOSOME TRANSFECTION | NANOPARTICLE TRANSFECTION | LIPID-BASED TRANSFECTION | POLYMER-BASED TRANSFECTION | USES OF DNA TRANSFECTION