LIPID TRANSFECTION
Transfection is the introduction of genetic material into a cell. Typically, the genetic material (DNA or RNA) is foreign to the cell and is introduced in order to produce a desired outcome. Most often, this is performed to eukaryotic cells (animal, plant, and fungus cells). Lipids are a type of molecule present in the cell that can create many different organic structures including fats, vitamins and the membrane surrounding organelles. Lipid-based transfection utilizes liposomes to attach to the cell and deposit a desired product.
Liposomes consist of phospholipid bilayers with a spherical shape. Their unique organization enables them to serve as tiny carriers of therapeutic drugs. These small, unilamellar microcontainers are used as carriers for therapeutic drug applications in both pre-clinical and clinical settings for cancer, infections and genetic diseases.
Liposomes deliver their cargo to cells in one of these manners:
- Endocytosis: Transport of particles into a living cell by invagination of the membrane
- Membrane Fusion: Two lipid bilayers join together to form a single entity
- Adsorption: To gather a liposome on the outside surface of a membrane
LIPID-BASED COMPLEXES
Liposomal transfection of cancer cell lines in cell biology research involves use of synthetic molecules that mimic the cellular membrane. These artificial liposomes are common with those found in the cell’s membrane, including hydrophilic properties, spherical form and ability to fuse with the membrane. Liposomes are capable to act as a delivery vehicle for nucleic acids, siRNAs (to induce RNAi), plasmid DNA (to express a gene of interest and/or alter the host cell genome) and multiple other applications. The charge of the liposome determines its attraction to the cell’s membrane and fusion interaction for cargo to be absorbed by the cell and released into its cytoplasm.
Cationic lipid formulations (see in vivo lipid-based transfection kits) have been successful in traversing the barriers into the cell, once assembled into an effective cationic lipid/DNA complex. A major benefit of liposome-formulated transfection reagents is that they are biologically inert and biocompatible; thus, they do not yield any unwanted cytotoxic effects.
LIPOSOME STRUCTURE
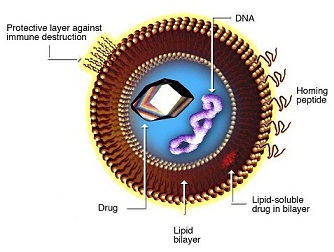
Basic liposome structure consists of a phospholipid bi-layer with an inner core. Both of these aspects can be customized for specific purposes.
Phospholipid bi-layer:
- Hydrophilic and hydrophobic regions enable protection for inner core payload
- Targeting and tissue specificity moieties can be added to the lipid-soluble layer, such as PEG or antibodies
- Can contain pH sensitive lipids that aid in cytoplasmic release of the active pharmaceutical ingredient (API) upon endocytosis
Inner core:
- Aqueous core enables encapsulation of hydrophilic nucleic acids and small molecules
- Can contain any of the following genetic material: RNA, DNA, siRNA, miRNA, aptamers, DNAzymes)
LIPID-BASED IN VIVO TRANSFECTION PROTOCOL
LIPID-BASED LIPOSOME DESCRIPTION
Lipid transfection reagent is a cationic lipid liposome-based formulation optimized for in vivo delivery of small RNA (siRNA, shRNA, microRNA) and plasmid DNA. LIPID in vivo transfection reagent is a proprietary, animal origin-free formulation.
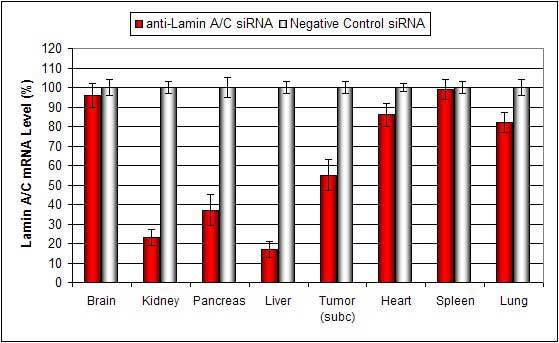
Lipid conjugated complexes are stable in serum for at least 16 hours. There is efficient delivery to the liver, pancreas, kidney, and certain tumor types via systemic administration. siRNA and plasmid DNA can also be injected via subcutaneous tumor injection with minimal toxicity.
Modes of Administration: Tail vein systemic intravenous (i.v.) injection. Direct intratumoral (i.t.) injection. Intraperitoneal (i.p.) injection.
LIPID-BASED SYSTEMIC ADMINISTRATION
Below is a protocol for the intraperitoneal or intravenous injection of lipid-based complexes for in vivo experiments.
- Dilute 100 µg of siRNA/miRNA or 60 µg of plasmid DNA in 100 µL of nuclease-free water and vortex gently.
- Add 100 µL of the diluted nucleic acid to a sterile tube containing 50 µL Transfection Reagent.
- Allow the complexes to incubate for 15-20 min at room temperature.
- Add 10 µL of the Transfection Enhancer Reagent to the tube and vortex gently to mix.
- Incubate for 5 min at room temperature.
- Add required amount of sterile solution of 5% glucose (w/v):
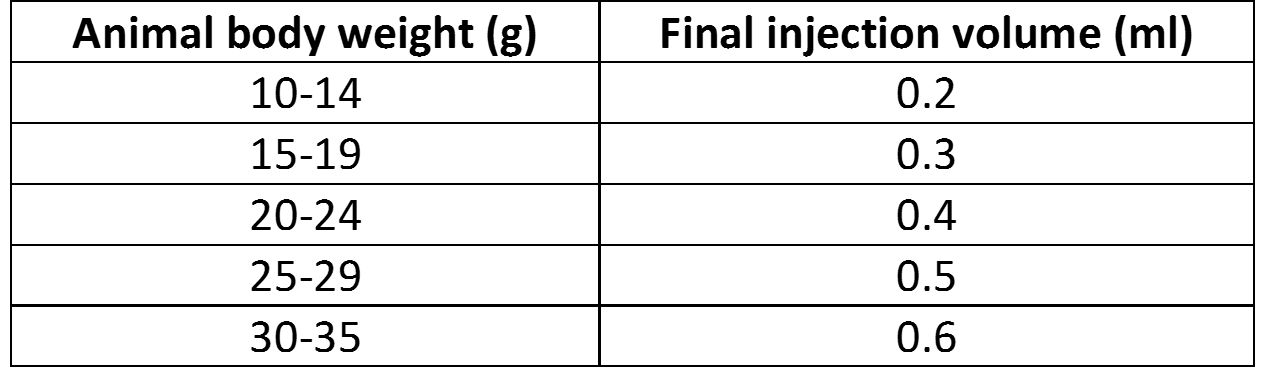
- Inject animals; optional: delivery efficiency can be increased with a secondary injection.
- Efficacy: Maximum mRNA target effect is observed 12-36 hours after injection, with maximum effect on protein level achieved 24-48 hours post-injection.
IN VIVO TRANSFECTION | PEG LIPOSOME TRANSFECTION | NANOPARTICLE TRANSFECTION | LIPID-BASED TRANSFECTION | POLYMER-BASED TRANSFECTION | USES OF DNA TRANSFECTION